911 Shirts – Thanking and Remembrance
On September 11, 2001, the world witnessed one of the most tragic and devastating acts of terrorism in modern history. On 9/11 Day every year, we gather to commemorate. We express our gratitude and keep their memory alive by wearing 9/11 shirts, that serve as symbols of thanks and remembrance. Through memorial services, moments of silence, acts of remembrance, we pay tribute to the victims of that fateful day.
What Is the Meaning of 911 Shirts?
Remembering the Heroes
The shirts offer a tangible way to honor their bravery, express our gratitude to the countless people who selflessly risked their lives to rescue and protect others who rushed into danger on that miserable day. They often feature powerful designs and symbols, such as the Twin Towers, American flags, and depictions of first responders. By wearing them, we demonstrate our appreciation for their selflessness and bravery, while keeping their memory alive.
Expressions of Gratitude
These 9/11 never forget shirts serve as a heartfelt expression of gratitude to the heroes. They allow us to honor the firefighters, police officers, paramedics, and other emergency personnel who ran towards the chaos without hesitation and the people who provided support and aid in the aftermath of the attacks. Additionally, the shirts acknowledge the passengers on United Airlines Flight 93 who valiantly fought against the hijackers, preventing further devastation. Wearing these shirts is a way to say “thank you” to those who put their lives on the line to protect others. (more…)
Fashion Secrets to Minimize the Appearance of Cellulite
Cellulite is a common cosmetic concern that affects people of all body types and sizes. While there’s no foolproof way to eliminate cellulite entirely, there are fashion secrets and styling techniques that can help minimize its appearance. By choosing the right clothing styles, fabrics, and accessories, you can boost your confidence and feel more comfortable in your own skin. In this article, we will explore some fashion secrets to help you reduce the visibility of cellulite and feel fabulous in any outfit.
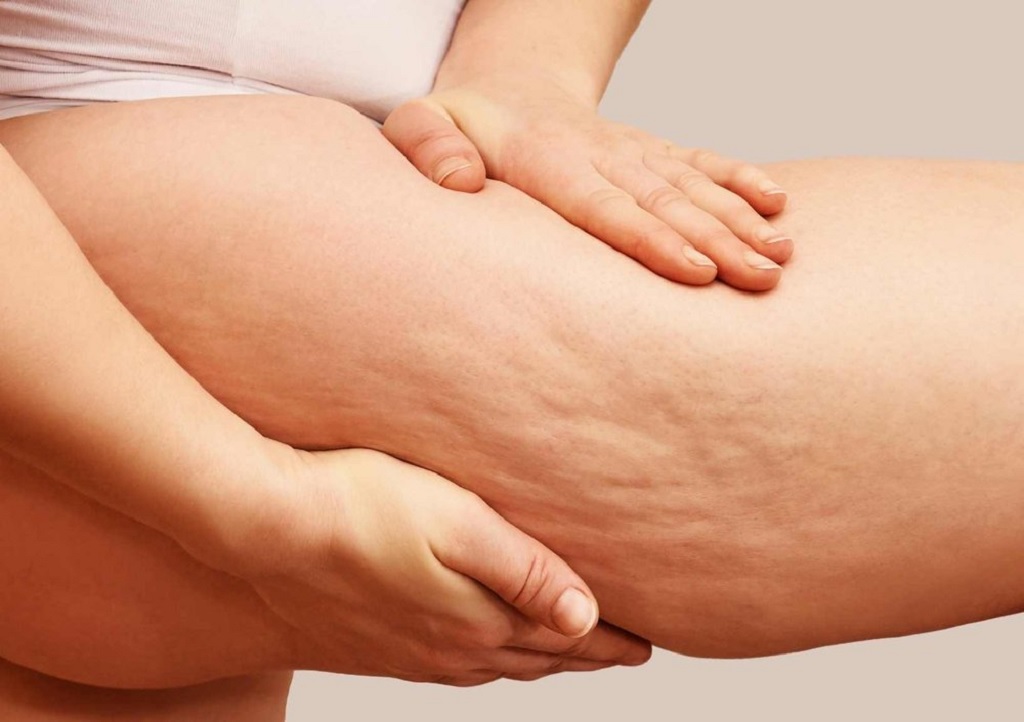
Fashion Tips to Disguise and Embrace Your Body
Opt for High-Waisted Bottoms
High-waisted bottoms, such as skirts, pants, and shorts, can work wonders in minimizing the appearance of cellulite. They provide coverage and support to areas prone to cellulite, particularly around the hips, thighs, and buttocks. By accentuating your waistline, high-waisted bottoms draw attention away from cellulite and create a more balanced silhouette. Best Lululemon pants for cellulite is an option you should try, Many Lululemon pants provide a compression fit, which can help to smooth and firm the skin’s appearance. The compression fabric hugs the body, creating a streamlined silhouette and potentially reducing the visibility of cellulite.
Embrace A-Line Dresses and Skirts
A-line dresses and skirts are another excellent choice for camouflaging cellulite. The gentle flare of these garments adds volume to the lower body, helping to disguise any lumps or bumps. Opt for A-line dresses that fall just above or below the knee to elongate your legs and divert attention from cellulite-prone areas. Pair them with heels to further enhance the overall slimming effect. (more…)
Nine Beautiful SNS Dip Colors for National Color Day
Life without color would be unimaginable. We use color in our lives every day. So, it is natural for the world to celebrate National Color Day. This year, National Color Day falls on October 22. Therefore, let us celebrate National Color Day with the best SNS dip powder colors on our nails. We shall discuss nine beautiful colors that signify various emotions. So, accordingly, we can choose our favorite nail colors on this day.
Favorite Colors for National Day
Here are nine exciting color options for National Color Day and their emotional significance.
Red – Love, Strength, and Excitement

Red is the most beautiful color to wear on your nails on National Color Day. This exciting shade signifies love. No wonder lovers like to wear bright reds on their nails to match their beautiful dresses and attract their boyfriends. Besides, red is a symbol of strength and dominance. So, if you love the excitement in your lives, red is the color to go to. (more…)
Know Why the Nail Activator Is a Critical Ingredient of the Dip Powder Kit
Dip powder manicure is one of the favored manicures today, with more young women preferring to have it. So, if you are familiar with dip nails, you must have heard about the activator and its significance in a dip powder manicure. What is the activator in a dip powder kit, and what is its importance? Let us discuss these aspects in detail in this article.
What Is a Dip Powder Nail Activator?

What separates these new nail manicure techniques like dip powder, acrylic, and gel polish from the traditional nail polish? First, all these manicures last longer than conventional nail polish. Besides, these new-age manicures have greater luster and look more attractive.
The activator is a crucial ingredient of the dip powder manicure, similar to the UV lamp we use for gel nail procedures. The nail activator is indispensable whenever you decide to have OPI dip nails because it helps the dip powder bond well with the basecoat and thus strengthens the manicure.
Everyone knows that UV exposure is essential to cure gel polish. Similarly, the activator is necessary for a dip powder manicure. The activator is an integral ingredient of the OPI dip powder kit. It comprises a clear liquid and is applied similarly to regular nail polish. In addition, the liquid activator contains chemical elements that help the dip powder stick to the basecoat and form chemical bonds that contribute to the manicure’s longevity. (more…)
How to Take Proper Care of Your Skin Before and after Getting Tattoos
Tattoos used to be considered a mark of rebellion, a way to stick it to the man. But in recent years, tattoos have become mainstream, and more and more people are getting them. That’s because tattoos can be beautiful, creative expressions of who you are. They can also be a source of pride and joy. But if you want your tattoos to look good for as long as possible, you need to take care of them properly. Here are some tips on how to take care of your skin before and after getting tattoos.
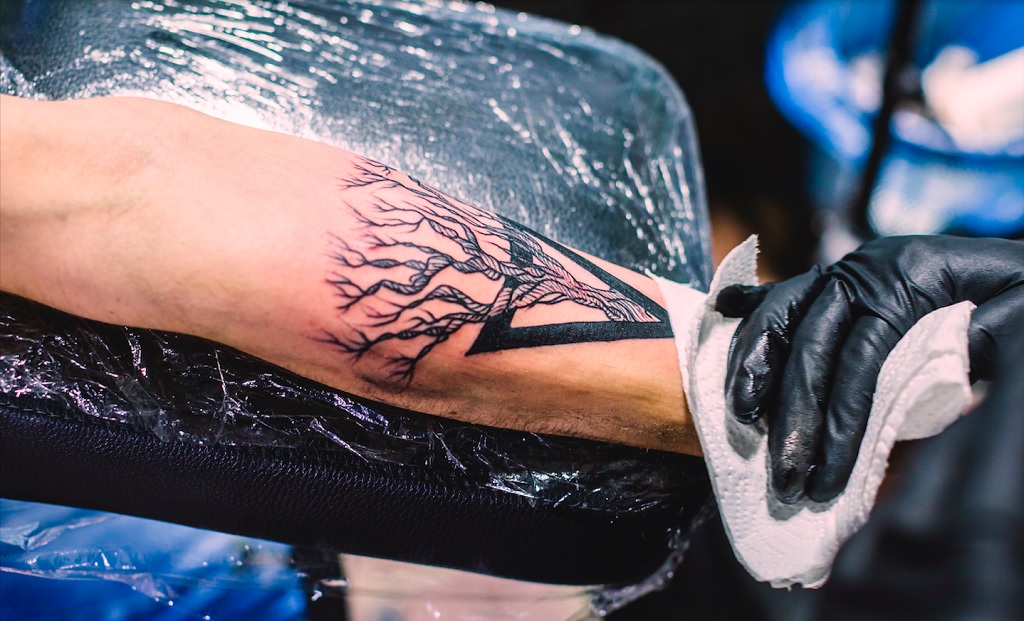
Before Getting a Tattoo
Do Not Use Blood Thinners
Do not use blood thinners before getting a tattoo as they can lead to excessive bleeding during the tattoo process. In addition to this, if you are taking any other medications, consult your doctor before getting a tattoo in order to avoid any inconvenience.
Use Numbing Cream to Prevent Pain
Some people find the pain of getting a tattoo to be unbearable. If you’re one of them, you can use a numbing cream to help reduce the pain. Just make sure to apply the best tattoo numbing cream before getting your tattoo appointment. It will make sure you do not feel any pain and discomfort during the process.
After Getting Tattoo
Keep Them Clean
One of the most significant things you can do to take care of your tattoos is to keep them clean. This means washing them regularly with soap and water. Make sure to use a gentle soap that won’t irritate your skin. You can also use a soap or cleansing product specifically designed for tattoos. (more…)
How to Choose Your Nail Polish to Suit Your Outfit
There is no doubt that your outfit defines who you are. However, you should also know that the nail polish you choose has a tremendous bearing in adding to the attraction quotient. That does not mean you should match your outfit, like red nail polish for a red dress, etc. We shall look at different ways to pick your nail polish to suit your outfit and the occasion.

So, it does not matter which color outfit you wear. You can find a nail polish color to suit it accordingly. OPI nail polish offers you an exciting range of OPI colors for you to choose from.
Best Nail Polish to Suit Your Outfit
Color blocking is a good idea
Color blocking is an excellent idea if you need to match your outfit with your nail polish. For example, you have colors like red and green or blue and yellow that look contrasting. However, they combine beautifully and look fabulous when you pair them together. (more…)
Choose Your DND Nail Colors According to Your Outfit
Nail polish serves as the perfect embellishment to your nails and makes them look attractive. The factors that affect the beauty quotient include your skin tone, the occasion, the season, and the outfit. Despite all these factors, picking the right nail colors is never easy. So let us understand how your outfits affect the nail colors you choose.
Matching Your Nails to Your Outfits Is Crucial

You should understand that your nail polish colors need not match perfectly with every outfit you wear. It is also difficult to match your nail polish colors with your dresses every time. Therefore, coordinating your nail colors with your outfits is critical. By coordinating your colors, you choose colors that match multiple outfits. Just as you wear outfits to suit the occasion, you should paint your nails according to the outfits you wear.
Let us now see which nail colors suit your different outfits.
The Interview Outfit
An interview is the most formal occasion you ever encounter in your life. Therefore, it is natural for your nails to look perfect for the interview. Though it can be tempting to wear deep reds to look beautiful, it is better to wear nudes and pastel colors to suit the interview attire.
The point is that your nails should be attractive but should not overshadow your personality. The deep reds look very dominant in your interview attire. Hence, you should avoid them and go for the nudes.
Secondly, you should avoid having multiple DND nail colors on your nails when matching with your interview attire. A better option is to go monochromatic. For example, if you wear an all-black dress for your interview, going for black DND nail gel polish should be the perfect match. You can also try out the grayish tones or the silvery tones, but having a contrasting white should not be on your agenda. If you love to have white, you can go for the French manicure with white or creamy tips. (more…)
A Complete Skincare Routine to Be Done at Different times of the Day
There is a common conception that skincare routines are meant to be done at night. This is what all influencers on social media try to portray. While night skincare routines are always beneficial and no one is denying that, it’s not a compulsion that you do your skincare only at night. It can be done at any time during the day and there are a number of products that are formulated to work at a particular instant in the 24 hours. In this guide, we’ll talk about all the skincare steps you can perform at various times during the day and get a beautiful, healthy skin.
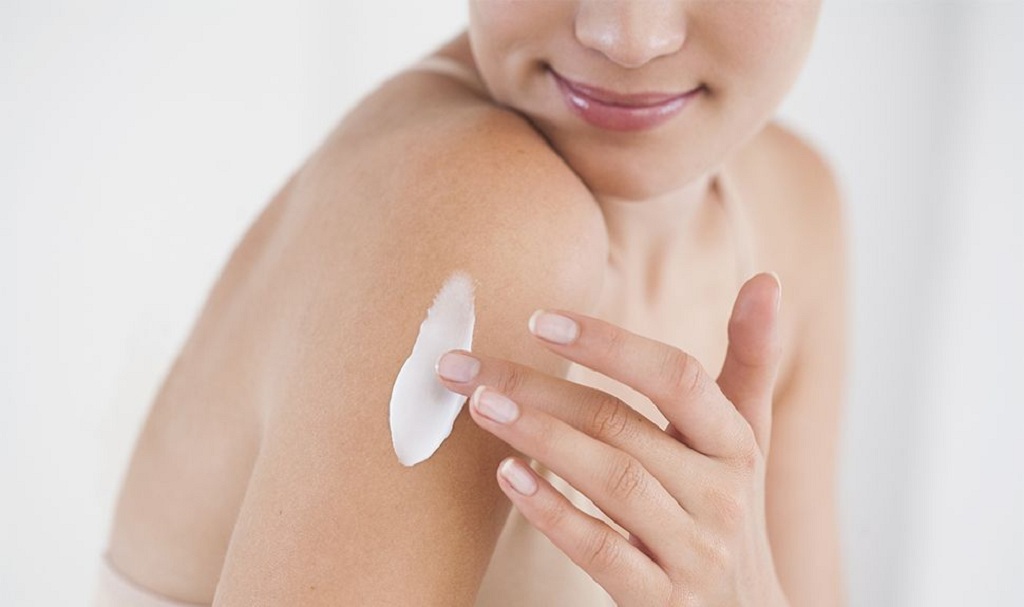
First thing to do in the morning
Getting up from a nap, the first thing you should do is to wash your face. During the night, a lot of oils accumulate into your skin especially if your skin is oily. These are supposed to be washed off and cleansed properly. You can try out a mattifying and gentle face wash or cleanser for this purpose.
Similarly, if you have dry skin, it may show signs of flakes and dry patches in the morning. So, for your face you should use a good foaming and gentle cleanser. For your body, choose the best soap for dry skin to wash off skin and make it softer. This step will preserve the natural balance of hydration, making your skin soft and nourished. (more…)
How to Use Your Gel Nail Starter Kit to Get a Beautiful Manicure
Well-manicured nails are beautiful to look at. However, the beauty gets accentuated by a coat of high-quality gel nail polish. It makes the nails noticeable and lets the woman make a powerful fashion statement. Do you wish to learn how to acquire a beautiful gel nail manicure? Read on to understand the gel manicure process that you can very well master at home.

Step by Step Apply Gel Nail Starter Kit
Get your nail starter kit ready
The first step towards acquiring the dream gel manicure is to get your nail starter kit ready. If you have one in your home, it should be fine. Otherwise, the OPI gel nail starter kit is easily available online. In addition, you can order your kit from us at reasonable prices. (more…)
The Wonders Face Masks Can Do for People with African American Skin Type
Face masks are very effective for a number of skin types, tones and conditions. Due to their large amount of benefits for the skin, they are one of the most highly recommended skincare products. The world’s most top ranking brands are producing the best face masks to cater the needs of every individual.
Talking about African American skin type particularly, it has some special needs. It is not at all an ordinary skin type. In this article, we’ll discuss how a face mask can benefit different skin types and African American skin types in general. Let’s get into all the basic information you need to know about this bundle of wonders.

Benefits of face masks
Face masks have a number of benefits. They give you the best type of skin treatment and are necessary to be a part of every skincare routine. Some benefits enlisted below:
- Provide hydration to the deepest skin cells
- Penetrate deeply and nourish the skin
- Treats open pores
- Works on skin elasticity and aging problems
- Ideal for oil and sebum control
- Essential to control acne and deep cleanse the skin
- Beneficial for avoiding pigmentation and dark spots
- Under eye bags and dark circles can be duly treated
Why this skin type need face masks
All skin types generally, and African American skin types particularly need a face mask for regular use. Here’s the reason why it is necessary.
This skin type is generally dry and flakes out quickly. A face mask can help in the restoration of water balance.
Dark skin is normally deficient in important skin components like vitamin C and folate. A face mask can uplift the nutrient supply and fulfill the needs of the skin.
African American skin types have rich deposits of melanin in their cells. This is the reason that their skin tone appears uneven and patchy. The use of a face mask for uneven skin tone can help clear out pigmented deposits. As a result you can get a more even and beautifully glowing skin. (more…)